Featured news
All news

You Make a Difference - February 2026
Every day, across our hospital, extraordinary people go above and beyond – not for recognition, but because they care. The You Make a Difference Awards shine a light on those moments of compassion, courage and commitment that truly change lives.

Clinical Engineering expert Professor Paul White wins AHCS Honorary Fellowship
An Addenbrooke’s professor who won a prestigious award at the beginning of the year has today won another top accolade for his work.
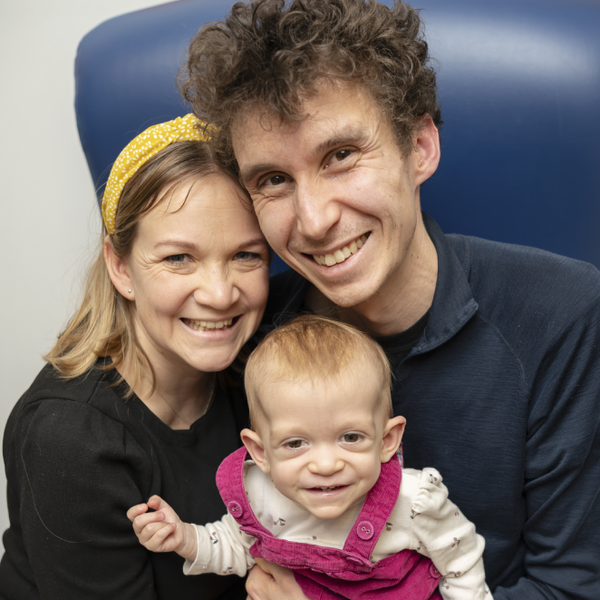
Genomic study helps detect baby’s rare growth condition
Baby Safi started treatment for a rare growth condition after participating in the Generation Study, a national genomic screening study

Global bone cement supply shortage - Update 25/02/2026
There has recently been a global shortage of medical bone cement, a material used in some orthopaedic procedures such as joint replacements. (Updated: Wednesday, 25th February 2026)

Update on our action plan: three-month milestones
We are publishing this update on the progress we have made against the commitments in our action plan ‘Learning, accountability and change'.

Study explores emerging evidence for greater sun awareness among drivers and passengers
A team at Addenbrooke’s Hospital has published a study exploring whether there is a link between skin cancer found on the right side of the face and head – and the side of the road on which this nation drives.

You Made a Difference - January 2026
Every day, across our hospital, extraordinary people go above and beyond – not for recognition, but because they care. The You Made a Difference Awards shine a light on those moments of compassion, courage and commitment that truly change lives.

Cutting-edge clinical training centre will have global reach
A Cambridge-based centre for training future healthcare professionals has doubled in capacity to become one of the most technically advanced in the country. Officially unveiled today, the Cambridge Digital Health and Surgical Training Centre now has an additional floor supporting immersive tuition using extended reality and Artificial Intelligence.

Prestigious fellowship for Cambridge cancer detection pioneer
Professor Rebecca Fitzgerald has been elected a fellow of the prestigious American Association for Cancer Research (AACR) Academy in recognition of her work on the capsule sponge – a quick and simple test that aids early detection of oesophageal cancer.

Study aims to help identify babies in intensive care who would benefit from additional support to prepare them for school
The BLOOMS study will use many tools to understand why some babies that spend time in NICU struggle starting school.

Paralympian Steve is first guest on research centre podcasts
Patients and others with an interest in brain and spinal injury could benefit from a fortnightly series of inspiring podcasts due to be aired this year by the NIHR HealthTech Research Centre (HRC) based at Addenbrooke’s.

You Made a Difference - December 2025
Every day, across our hospital, extraordinary people go above and beyond – not for recognition, but because they care. The You Made a Difference Awards shine a light on those moments of compassion, courage and commitment that truly change lives.

Targeting the immune system could prevent repeat heart attacks, trial suggests
A study of 60 people suggests how targeting the immune system could prevent people who have recently had a heart attack from having more of them.

Transplant pioneer recognised in New Year Honours
An honorary consultant and keyhole surgery pioneer at Addenbrooke’s Hospital has been recognised in the New Year’s honours list.

Update: Fire in car park 1
Car Park 1 has now safely reopened following the recent fire, and NCP is carrying out repair works while we continue to provide as much parking capacity as possible for our staff, patients and visitors.

Behind the scenes: Stand Up To Cancer live from Addenbrooke’s
Last week a special programme for Stand Up To Cancer presented by Davina McCall was broadcast live from Addenbrooke’s Hospital.

Addenbrooke's prepares for live Stand Up To Cancer programme tonight
Cancer Clinic Live will be broadcast on Channel 4 from 8pm on Friday 12th December.

Resident doctor's industrial action - 17 December - 22 December 2025
The British Medical Association (BMA) has confirmed industrial action during December 2025.

Park & ride solar panels boost hospital green plan
Solar panels installed at Babraham Road Park & Ride have been successfully connected to the energy grid and are creating green electricity to power electric vehicles and Addenbrooke’s Hospital.

High-tech training centre prepares surgeons for future
The next generation of young surgeons attending a Cambridge-based training centre are set to benefit from more world-class facilities – and pioneering tuition in a fast-growing area of healthcare.